Launch Time: 2016-12-18 Views: 1868 Rely: 0 Started by:
00e5da80.jpg?w=225&h=225)
The nicotine component, whether prefilled cartridges or bottles of e-liquid, where it is high enough to be classified under CLP (i.e. 2.5% and over), must be supplied in child-resistant packaging, with:
a) a replaceable closure unless the packaging complies with the requirements of BS EN 28317, or ISO 8317; or
b) a non-replaceable closure unless the packaging complies with the requirements of EN 862. (Given the wide variety and quality of ‘child-resistant’ closures available, ECITA members should submit samples for assessment and expert opinion); and
c) must be appropriately labelled as follows:
The exact warnings and markings depend on the level of nicotine, as indicated below. (Please note that you are not required to provide the statement codes, e.g. ‘H300’ on your labels. These are included here to assist you with finding the appropriate translations of these statements into other Member State languages for exports across Europe. Please see the tables for all the languages for hazard statements(3), and notes on the precautionary statements(4) in English.)
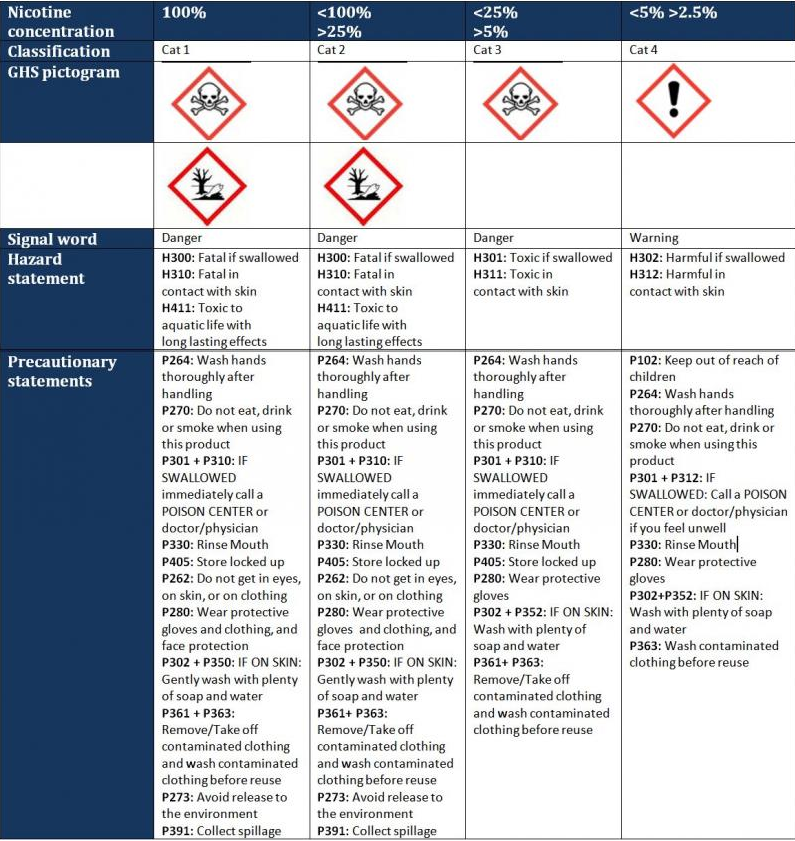
The text of the hazard and precautionary statements can be found in table 1.2 of the CLP Regulation (on page 165 onwards, in the current version), which should enable appropriate terms to be used to suit the Member State where the product is to be supplied.
A tactile warning label, compliant with EN ISO 11683, should be affixed and formed as follows:
a tactile label must be a raised equilateral triangle which must have its corners as sharp as possible; it must be in the form of a frame and have sides 16 – 20mm long, 1.5 – 1.9mm thick, raised 0.25 – 0.5mm above the surrounding surface and not more than 1mm apart at the corners; If the tactile label is smaller, it must be:
a smaller triangle: as above, but the frame may be filled, and the sides must be 8 – 10mm long and if not filled 0.8 – 1.2mm thick;
a very small triangle: as above, but the frame must be filled and the sides must be 3 – 4mm long.
Alternatively:
3 dots, each with a truncated cone shape, equally spaced on a circle with each dot having a diameter of 1.8 – 2.2mm, having a height of 0.25 – 0.5mm and being 3 – 9mm apart (centre to centre).
Tactile labels should be located so that any other embossed patterns do not cause confusion and, where the packaging has a bottom:
on the upright handling surface near the edge;
that its apex is within 50mm of the bottom of the pack (or as near as possible to the lid if there is no bottom);
Except that (unless it is technically impossible) when the warning is:
on a surface which is removed during normal use;
on plastic packaging with full opening (injection process) – it must be on the handling surface as near as possible to the opening.
Where the packaging does not have a bottom:
(e.g. tubes and cartridges) on the shoulder around the tube nozzle, in the form of dots and triangles evenly spaced with each triangle pointing towards the outside of the tube;
(other packaging) on the handling surface at the manufacturers’ discretion. These must remain tactile throughout the product’s expected life.
• The person who first supplies a dangerous preparation must keep a record of the details of any child-resistant fastening or tactile warning for at least 3 years after it was supplied for the last time, and must make it available on request to an enforcement officer within 14 days, along with any certificate issued by a qualified test house in relation to that fastening or tactile warning. We can offer this service for non-member companies. Please contact us for details.
• It must clearly state on the label, and on the outer packaging if appropriate (sample labels are provided to ECITA members), that the product contains nicotine;
• The nominal quantity of the solution contained within the bottle/package (see ANNEXES X AND XI) must be displayed;
• The EC number for nicotine (EC 200-193-3) should be displayed on the label, and on the outer packaging if appropriate;
• The nicotine content level, as a percentage of weight by volume must be clearly displayed, and must not exceed 7.5%. (Alternatively, it may be displayed as mg/ml, although this can be misleading, so ECITA does not recommend this practice.);
• The batch number or code;
• The supplier’s name, physical address (not a PO Box or simply a web address/email) and contact telephone number (landline, not just mobile);
• Country of origin, displayed as “Made in...”; and
• The trade name or other designation of the product.
• The above information must be clearly and indelibly marked on the label of the receptacle containing the nicotine solution. The label must be securely attached, with its entire surface making contact with the bottle/package;
• if the receptacle is supplied in outer packaging, such as a box, then the outermost layer must be similarly clearly and indelibly marked, unless the packaging allows the receptacle label to be clearly and legibly seen;
• the colours of the label must be such that the hazard symbol and the risk and safety phrase wording stand out clearly from the background;
• the wording must be of sufficient size and spacing to allow it to be easily read, and must read horizontally when the package is set down normally;
• the hazard pictogram must be of suitable size, and must not be less than 100mm2; this is of particular relevance where bulk containers are used;
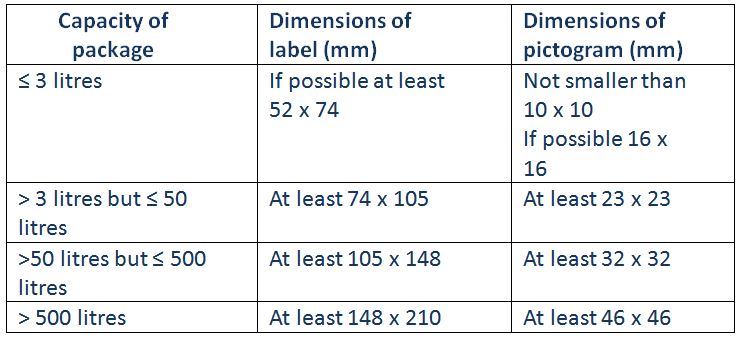
• if the package is too small or awkward in shape for a label of the right size to be attached completely, it may be affixed in some other appropriate manner;
• a Safety Data Sheet in addition to a label on each single product must be provided when selling in bulk or wholesale to people other than members of the public, e.g. to distributors or resellers; and
• ECITA requires that its members also take all necessary steps to ensure that their products are not sold to minors, including displaying relevant information on the label, and outer packaging if appropriate.
The Safety Data Sheet for an e-liquid will not be the same as for nicotine, propylene glycol or glycerol on their own, since e-liquid is a mixture. Template SDS sheets are provided to ECITA members, but we can offer assistance to non-members, too, for a modest fee. Please contact us for details.